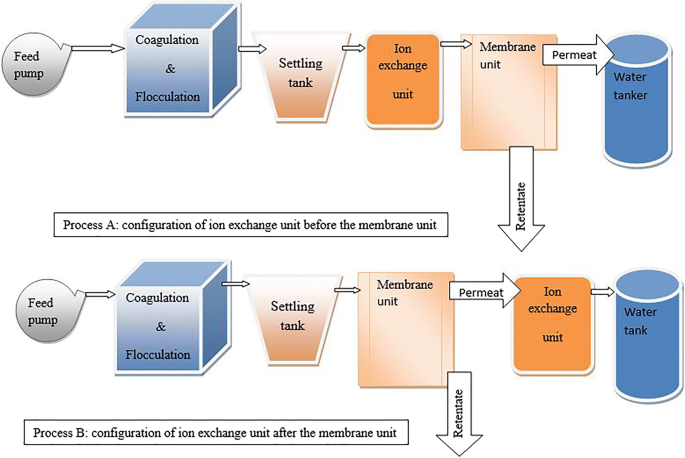
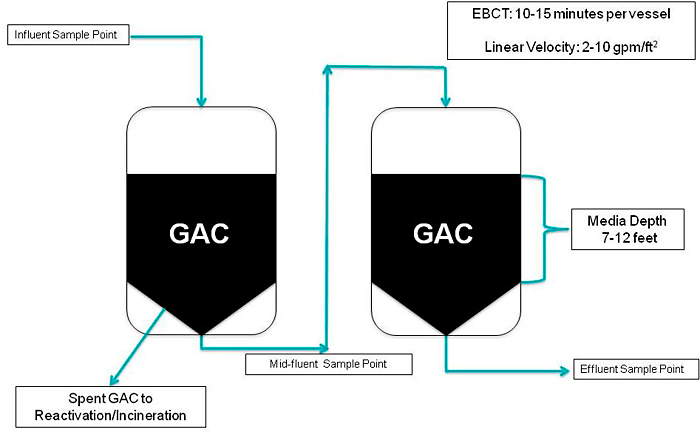
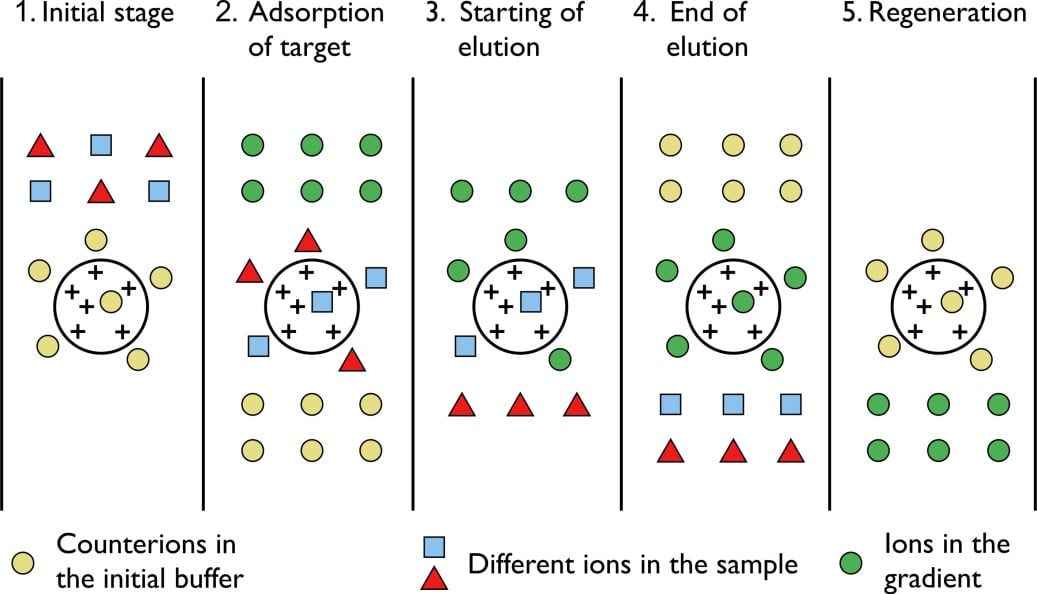
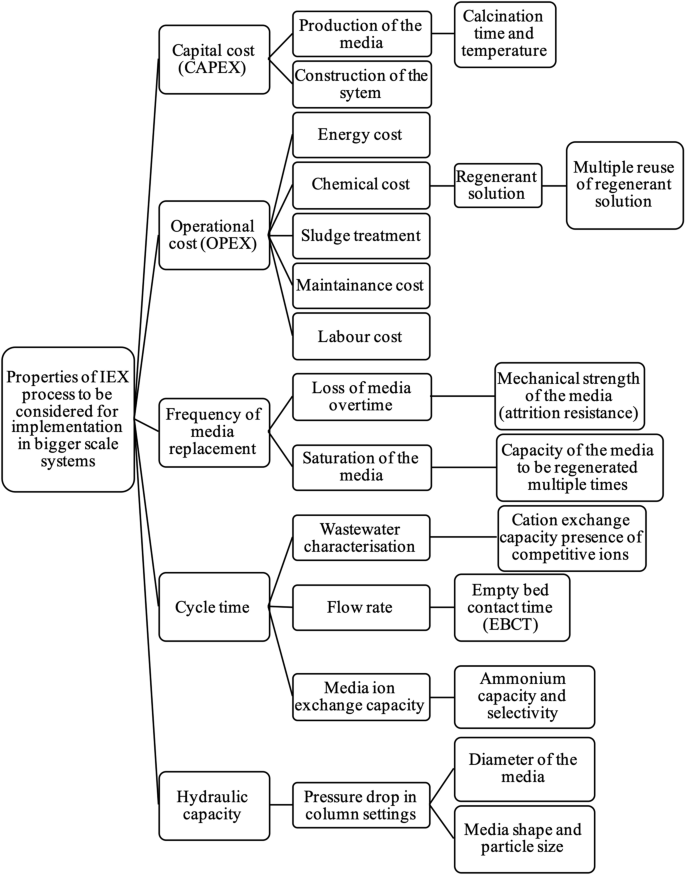
Ion exchange is a water treatment commonly used for water softening or deminerialization and to remove other substances from water.
Ion exchange process in water treatment pdf.
Choosing and using ion exchange resins for water treatment systems is often a perplexing pro cedure.
This ability is also seen in various natural.
It has special utility in chemical synthesis medical research food processing mining agriculture and a variety of.
Plant engineers not familiar with such resins encounter a strange array of nomenclature and marketing practices that imply.
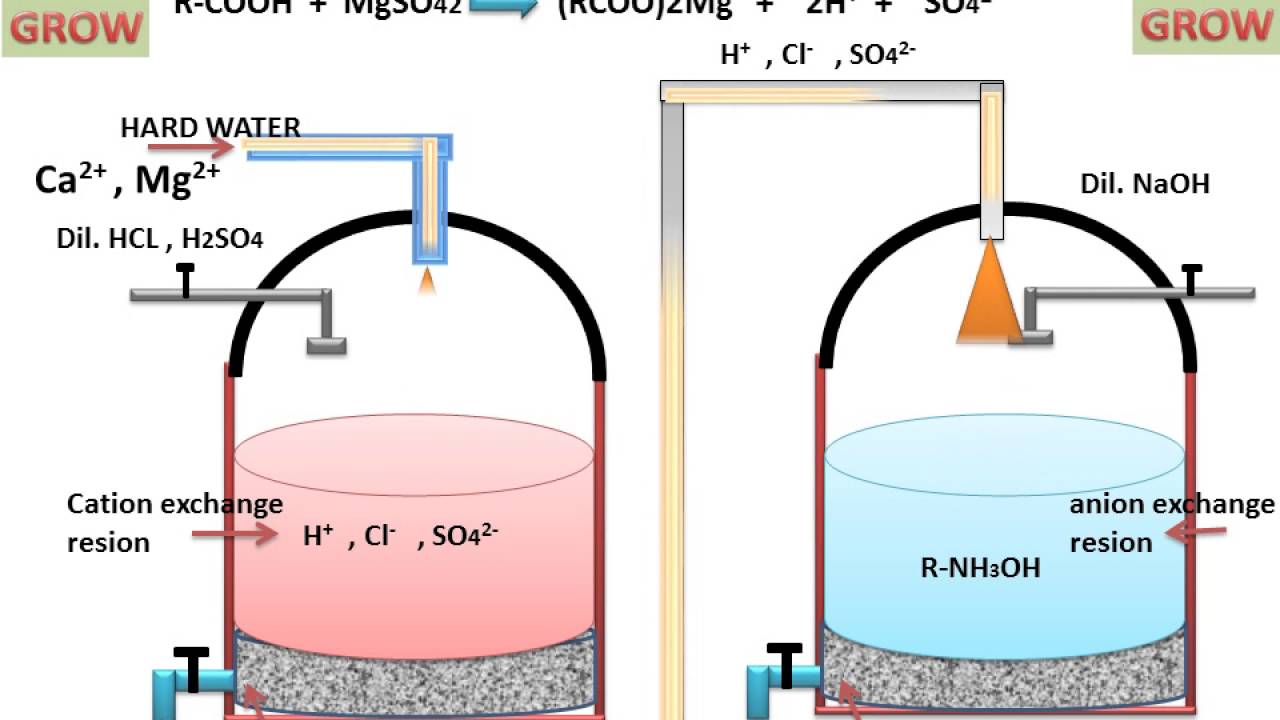
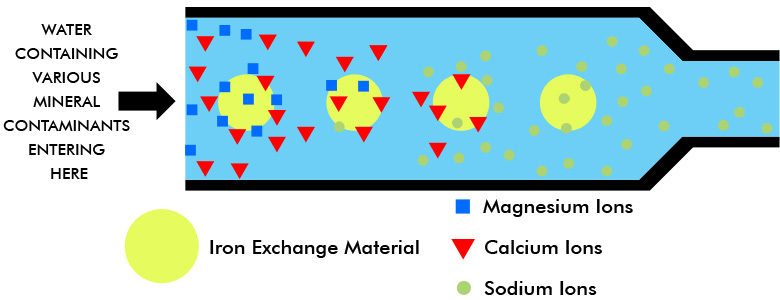
Ion exchange resins are polymers that are capable of exchanging particular ions within the polymer with ions in a solution that is passed through them.
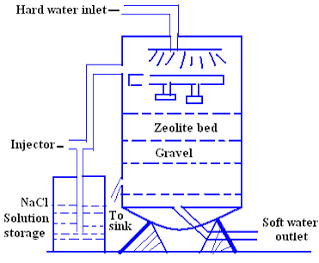
In the ion exchange process sodium ions are used to coat an exchange medium in.
Ion exchange processes can also remove various charged atoms or molecules ions such as nitrates fluoride sulphates perchlorate iron.
Ion exchange is used in water treatment and also provides a method of separation in many non water processes.
Although different water treatment products utilize this process in different ways the action itself remains the.
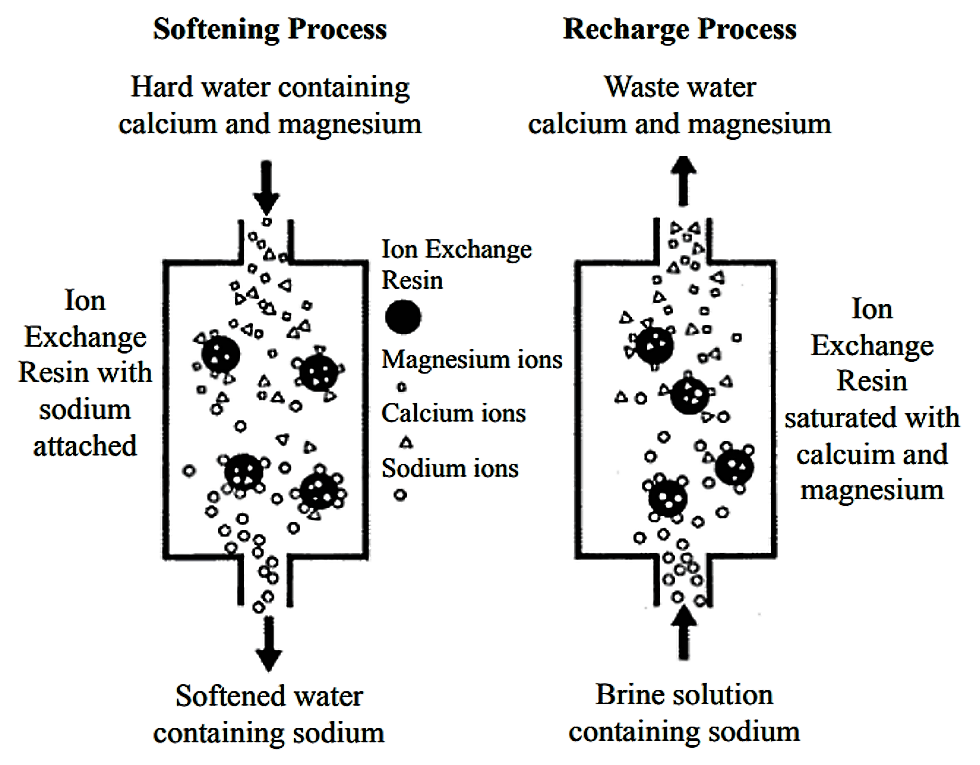
In the softening of water by the ion exchange process the calcium and magnesium ions are removed from the solution and the exchanger solid releases sodium ions to replace the removed calcium and magnesium 2rna ca 2.
An ion is an atom or.
This guide discusses the ion exchange water softening process and related equipment used for household water treatment.
Sodium ions are supplied from dissolved sodium chloride salt also called brine.
The ion exchange process can be used in a variety of ways when it comes to water purification and softening.
Hard water can be softened using an ion exchange softening process.
Ion exchange is a water treatment process commonly used for water softening or demineralization but it also is used to remove other substances from the water in processes such as dealkalization deionization and disinfection.